标准电极电势表,指的是半反应按电极电势由低到距劳兵侵以伯等片前高排序,可十分简明地判断氧化还原反应的方向。标准电极电势是可逆电极在标准状态及平衡态时的电势,也就是标准态时的电极电势。标准电极电势有很大的实用价值,可用来判断氧化剂与还原剂的相对强弱,判断氧化还原反应的进行方向,计算原电池的电动势、反应自由能、平衡常数,计算其他半反应的标准电极电势等。
- 中文名称 标准电极电势表
- 外文名称 Tables of Standard Electrode Potentials
- 表达式 E°(T)=-0.0003T+0.1414,T为温度,单位为K
- 适用领域 化学滴定分析,电池等
- 应用学科 电化学等
电极电势产生
德国化学家能斯特(H.W.Nernst)提出了双电层理论(electron double lay景很落啊外济却著次孔兵er theory)解释电极电势的产生的原因。当金属放入溶液中时,一方面金属晶体中处于热运动的金属离子在极性水分子的作用下,离开金属表面进入溶液。金属性质越活泼,这种趋势就越大;另一方面溶液中的金属离子,由于受到金属表面克根引统济甲电子的吸引,而在金属表面沉积,溶液中金属离子的浓度越大,这种趋势也越大。在一定浓度的溶液中达到平衡后,在金属和溶液两相界面上形成了一个带相反电荷的双电层(electron double layer),双电层的厚度虽六培孔程操术轻卫然很小(一般约为0.2-20纳米数量级), 但却在来自金属和溶液之间产生360百科了电势差。通常人们就把产生在金属和盐溶液之间的双电层间的电势差称为金属的电极电势(electrode potential),并以此描述电极得失电子能力的相对强弱。电极电势以符号已态E(M/M)表示, 单位为V(伏)。 如锌的电极电势以E(Zn/Zn)表示, 铜的电促红赶束女极电势以E(Cu/Cu)表示。
电极电势的大小主要映取决于电极的本性,并受温度支被苦、介质和离子浓度等因素的影响。
公式
任何温度下标准氢电极的标准电极电势值都为0,但其他电极电势值会受到温度影响。以Ni/NiO电极为例,它可以用作高温伪参比电极,在0-400°C时的电极电势大致符合以下公式:
E°(T)=-0.0003T+0.1414,T为温度,单位为K
表中数据多为298K(25℃ )条件
电极电势表
酸深条接性
电对 | 方程式 | E完助农标罗务程肉q/V |
Li(I)-(0) | 神妒酒首验脸抗针华 Li+e=Li | -3.0401 |
K(I)-(0) | K+e=K | -2.931 |
Rb(I)-(0) | Rb+e=Rb | -2.927 |
Cs(I)-(0) | Cs+e=Cs | 危跳左却立孙两青-2.925 |
Ba(II)-(0) | Ba+2e=Ba | -2.912 |
Sr(II)-(0) | Sr+2e=Sr | -2.89 |
Ca并镇星束合决(II)-(0) | Ca+2e=Ca | -2.868 |
Na(I)-(0) | 来自 Na+e=Na | -2.71 |
La(III)-(0) | La+谁起组呀状事波3e=La | -2.360百科379 |
Mg(II)-(0) | Mg+2e=Mg | -2.372 |
Ce(III)-(0) | Ce+3e=Ce | -2.336 |
者克苏机段如力命扬配 H(0)-(-I) | H2(g)+2e=2H | -2.23 |
Al(III)-(0) | AlF6+3e=Al+6F | -2.069 |
Th(IV)-(0) | Th+4e=Th | -1.899 |
Be(跑呢用围轻元II)-(0) | Be+2e=树客企Be | -1.847 |
价衣花宪历留英事干乎菜 U(III)-(0) | U+3e=U | -1.798 |
Hf(IV)-(0) | HfO+2H+4e=Hf+H2O | -1.724 |
Al(III)-(0) | 批号互素于酒地乎 Al+3e=Al | -1.662 |
Ti(II)-(0) | 云强绿陈克降给 Ti+2e=Ti | -1兵附观祖怕.630 |
Zr(IV)-(0) | ZrO2+4H+4e=Zr+2H2O | -1.553 |
Si(IV)-(0) | [SiF6]+4e=Si+6F | -1.24 |
Mn(II)国慢福约-(0) | Mn+2e=Mn | -1.185 |
影声油钱一核敌组Cr(II)-(0) | Cr+2e=Cr | -0.913 |
T上别友达喜菜委执翻回i(III)-(II) | Ti+e=Ti | -0.9 |
B(III)-(0) | H3BO3+3H+3e=B+3H2O | -0.8698 |
*Ti(IV)-(0氧长) | TiO2+4H温高混志皮族后+4e=Ti+2H2O | -0.86 |
Te(0)-(-II) | Te+2H+2e=H2Te | -0.793 |
Zn(II)-(0) | Zn+2e=Zn | -0.7618 |
Ta(V)-(0) | Ta2O5+10H+10e=2Ta+5H2O | -0.750 |
Cr(III)-(0) | Cr+3e=Cr | -0.744 |
Nb(V)-(0) | Nb2O5+10H+10e=2Nb+5H2O | -0.644 |
As(0)-(-III) | As+3H+3e=AsH3 | -0.608 |
U(IV)-(III) | U+e=U | -0.607 |
Ga(III)-(0) | Ga+3e=Ga | -0.549 |
P(I)-(0) | H3PO2+H+e=P+2H2O | -0.508 |
P(III)-(I) | H3PO3+2H+2e=H3PO2+H2O | -0.499 |
*C(IV)-(III) | 2CO2+2H+2e=H2C2O4 | -0.49 |
Fe(II)-(0) | Fe+2e=Fe | -0.447 |
Cr(III)-(II) | Cr+e=Cr | -0.407 |
Cd(II)-(0) | Cd+2e=Cd | -0.4030 |
Se(0)-(-II) | Se+2H+2e=H2Se(aq) | -0.399 |
Pb(II)-(0) | PbI2+2e=Pb+2I | -0.365 |
Eu(III)-(II) | Eu+e=Eu | -0.36 |
Pb(II)-(0) | PbSO4+2e=Pb+SO4 | -0.3588 |
In(III)-(0) | In+3e=In | -0.3382 |
Tl(I)-(0) | Tl+e=Tl | -0.336 |
Co(II)-(0) | Co+2e=Co | -0.28 |
P(V)-(III) | H3PO4+2H+2e=H3PO3+H2O | -0.276 |
Pb(II)-(0) | PbCl2+2e=Pb+2Cl | -0.2675 |
Ni(II)-(0) | Ni+2e=Ni | -0.257 |
V(III)-(II) | V+e=V | -0.255 |
Ge(IV)-(0) | H2GeO3+4H+4e=Ge+3H2O | -0.182 |
Ag(I)-(0) | AgI+e=Ag+I | -0.15224 |
Sn(II)-(0) | Sn+2e=Sn | -0.1375 |
Pb(II)-(0) | Pb+2e=Pb | -0.1262 |
*C(IV)-(II) | CO2(g)+2H+2e=CO+H2O | -0.12 |
P(0)-(-III) | P(白磷)+3H+3e=PH3(g) | -0.063 |
Hg(I)-(0) | Hg2I2+2e=2Hg+2I | -0.0405 |
Fe(III)-(0) | Fe+3e=Fe | -0.037 |
H(I)-(0) | 2H+2e=H2 | 0.0000 |
Ag(I)-(0) | AgBr+e=Ag+Br | 0.07133 |
S(II.V)-(II) | S4O6+2e=2S2O3 | 0.08 |
*Ti(IV)-(III) | TiO+2H+e=Ti+H2O | 0.1 |
S(0)-(-II) | S+2H+2e=H2S(aq) | 0.142 |
Sn(IV)-(II) | Sn+2e=Sn | 0.151 |
Sb(III)-(0) | Sb2O3+6H+6e=2Sb+3H2O | 0.152 |
Cu(II)-(I) | Cu+e=Cu | 0.153 |
Bi(III)-(0) | BiOCl+2H+3e=Bi+Cl+H2O | 0.1583 |
S(VI)-(IV) | SO4+4H+2e=H2SO3+H2O | 0.172 |
Sb(III)-(0) | SbO+2H+3e=Sb+H2O | 0.212 |
Ag(I)-(0) | AgCl+e=Ag+Cl | 0.22233 |
As(III)-(0) | HAsO2+3H+3e=As+2H2O | 0.248 |
Hg(I)-(0) | Hg2Cl2+2e=2Hg+2Cl(饱和KCl) | 0.26808 |
Bi(III)-(0) | BiO+2H+3e=Bi+H2O | 0.320 |
U(VI)-(IV) | UO2+4H+2e=U+2H2O | 0.327 |
C(IV)-(III) | 2HCNO+2H+2e=(CN)2+2H2O | 0.330 |
V(IV)-(III) | VO+2H+e=V+H2O | 0.337 |
Cu(II)-(0) | Cu+2e=Cu | 0.3419 |
Re(VII)-(0) | ReO4+8H+7e=Re+4H2O | 0.368 |
Ag(I)-(0) | Ag2CrO4+2e=2Ag+CrO4 | 0.4470 |
S(IV)-(0) | H2SO3+4H+4e=S+3H2O | 0.449 |
Cu(I)-(0) | Cu+e=Cu | 0.521 |
I(0)-(-I) | I2+2e=2I | 0.5355 |
I(0)-(-I) | I3+2e=3I | 0.536 |
As(V)-(III) | H3AsO4+2H+2e=HAsO2+2H2O | 0.560 |
Sb(V)-(III) | Sb2O5+6H+4e=2SbO+3H2O | 0.581 |
Te(IV)-(0) | TeO2+4H+4e=Te+2H2O | 0.593 |
U(V)-(IV) | UO2+4H+e=U+2H2O | 0.612 |
**Hg(II)-(I) | 2HgCl2+2e=Hg2Cl2+2Cl | 0.63 |
Pt(IV)-(II) | [PtCl6]+2e=[PtCl4]+2Cl | 0.68 |
O(0)-(-I) | O2+2H+2e=H2O2 | 0.695 |
Pt(II)-(0) | [PtCl4]+2e=Pt+4Cl | 0.755 |
*Se(IV)-(0) | H2SeO3+4H+4e=Se+3H2O | 0.74 |
Fe(III)-(II) | Fe+e=Fe | 0.771 |
Hg(I)-(0) | Hg2+2e=2Hg | 0.7973 |
Ag(I)-(0) | Ag+e=Ag | 0.7996 |
Os(VIII)-(0) | OsO4+8H+8e=Os+4H2O | 0.8 |
N(V)-(IV) | 2NO3+4H+2e=N2O4+2H2O | 0.803 |
Hg(II)-(0) | Hg+2e=Hg | 0.851 |
Si(IV)-(0) | SiO2(石英)+4H+4e=Si+2H2O | 0.857 |
Cu(II)-(I) | Cu+I+e=CuI | 0.86 |
N(III)-(I) | 2HNO2+4H+4e=H2N2O2+2H2O | 0.86 |
Hg(II)-(I) | 2Hg+2e=Hg2 | 0.920 |
N(V)-(III) | NO3+3H+2e=HNO2+H2O | 0.934 |
Pd(II)-(0) | Pd+2e=Pd | 0.951 |
N(V)-(II) | NO3+4H+3e=NO+2H2O | 0.957 |
N(III)-(II) | HNO2+H+e=NO+H2O | 0.983 |
I(I)-(-I) | HIO+H+2e=I+H2O | 0.987 |
V(V)-(IV) | VO2+2H+e=VO+H2O | 0.991 |
V(V)-(IV) | V(OH)4+2H+e=VO+3H2O | 1.00 |
Au(III)-(0) | [AuCl4]+3e=Au+4Cl | 1.002 |
Te(VI)-(IV) | H6TeO6+2H+2e=TeO2+4H2O | 1.02 |
N(IV)-(II) | N2O4+4H+4e=2NO+2H2O | 1.035 |
N(IV)-(III) | N2O4+2H+2e=2HNO2 | 1.065 |
I(V)-(-I) | IO3+6H+6e=I+3H2O | 1.085 |
Br(0)-(-I) | Br2(aq)+2e=2Br | 1.0873 |
Se(VI)-(IV) | SeO4+4H+2e=H2SeO3+H2O | 1.151 |
Cl(V)-(IV) | ClO+2H+e=ClO2+H2O | 1.152 |
Pt(II)-(0) | Pt+2e=Pt | 1.18 |
Cl(VII)-(V) | ClO4+2H+2e=ClO3+H2O | 1.189 |
I(V)-(0) | 2IO3+12H+10e=I2+6H2O | 1.195 |
Cl(V)-(III) | ClO3+3H+2e=HClO2+H2O | 1.214 |
Mn(IV)-(II) | MnO2+4H+2e=Mn+2H2O | 1.224 |
O(0)-(-II) | O2+4H+4e=2H2O | 1.229 |
Tl(III)-(I) | Tl+2e=Tl | 1.252 |
Cl(IV)-(III) | ClO2+H+e=HClO2 | 1.277 |
N(III)-(I) | 2HNO2+4H+4e=N2O+3H2O | 1.297 |
**Cr(VI)-(III) | Cr2O7+14H+6e=2Cr+7H2O | 1.33 |
Br(I)-(-I) | HBrO+H+2e=Br+H2O | 1.331 |
Cr(VI)-(III) | HCrO4+7H+3e=Cr+4H2O | 1.350 |
Cl(0)-(-I) | Cl2(g)+2e=2Cl | 1.35827 |
Cl(VII)-(-I) | ClO4+8H+8e=Cl+4H2O | 1.389 |
Cl(VII)-(0) | 2ClO4+16H+14e=Cl2+8H2O | 1.39 |
Au(III)-(I) | Au+2e=Au | 1.401 |
Br(V)-(-I) | BrO3+6H+6e=Br+3H2O | 1.423 |
I(I)-(0) | 2HIO+2H+2e=I2+2H2O | 1.439 |
Cl(V)-(-I) | ClO3+6H+6e=Cl+3H2O | 1.451 |
Pb(IV)-(II) | PbO2+4H+2e=Pb+2H2O | 1.455 |
Cl(V)-(0) | 2ClO3+12H+10e=Cl2+6H2O | 1.47 |
Cl(I)-(-I) | HClO+H+2e=Cl+H2O | 1.482 |
Br(V)-(0) | 2BrO3+12H+10e=Br2+6H2O | 1.482 |
Au(III)-(0) | Au+3e=Au | 1.498 |
Mn(VII)-(II) | MnO4+8H+5e=Mn+4H2O | 1.507 |
Mn(III)-(II) | Mn+e=Mn | 1.5415 |
Cl(III)-(-I) | HClO2+3H+4e=Cl+2H2O | 1.570 |
Br(I)-(0) | 2HBrO+2H+2e=Br2(aq)+2H2O | 1.574 |
N(II)-(I) | 2NO+2H+2e=N2O+H2O | 1.591 |
I(VII)-(V) | H5IO6+H+2e=IO3+3H2O | 1.601 |
Cl(I)-(0) | 2HClO+2H+2e=Cl2+2H2O | 1.611 |
Cl(III)-(I) | HClO2+2H+2e=HClO+H2O | 1.645 |
Ni(IV)-(II) | NiO2+4H+2e=Ni+2H2O | 1.678 |
Mn(VII)-(IV) | MnO4+4H+3e=MnO2+2H2O | 1.679 |
Pb(IV)-(II) | PbO2+SO4+4H+2e=PbSO4+2H2O | 1.6913 |
Au(I)-(0) | Au+e=Au | 1.692 |
Ce(IV)-(III) | Ce+e=Ce | 1.72 |
N(I)-(0) | N2O+2H+2e=N2+H2O | 1.766 |
O(-I)-(-II) | H2O2+2H+2e=2H2O | 1.776 |
Co(III)-(II) | Co+e=Co(2mol·LH2SO4) | 1.83 |
Ag(II)-(I) | Ag+e=Ag | 1.980 |
S(VII)-(VI) | S2O8+2e=2SO4 | 2.010 |
O(0)-(-II) | O3+2H+2e=O2+H2O | 2.076 |
O(II)-(-II) | F2O+2H+4e=H2O+2F | 2.153 |
Fe(VI)-(III) | FeO4+8H+3e=Fe+4H2O | 2.20 |
O(0)-(-II) | O(g)+2H+2e=H2O | 2.421 |
F(0)-(-I) | F2+2e=2F | 2.866 |
F(0)-(-I) | F2+2H+2e=2HF | 3.053 |
备注:
*摘自J.A. Dean Ed,Lange's Handbook of Chemistry, 13th. edition, 1985。
**摘自其他参考书。
碱性
电对 | 方程式 | Eq/V |
Ca(II)-(0) | Ca(OH)2+2e=Ca+2OH | -3.02 |
Ba(II)-(0) | Ba(OH)2+2e=Ba+2OH | -2.99 |
La(III)-(0) | La(OH)3+3e=La+3OH | -2.90 |
Sr(II)-(0) | Sr(OH)2·8H2O+2e=Sr+2OH+8H2O | -2.88 |
Mg(II)-(0) | Mg(OH)2+2e=Mg+2OH | -2.690 |
Be(II)-(0) | Be2O3+3H2O+4e=2Be+6OH | -2.63 |
Hf(IV)-(0) | HfO(OH)2+H2O+4e=Hf+4OH | -2.50 |
Zr(IV)-(0) | H2ZrO3+H2O+4e=Zr+4OH | -2.36 |
Al(III)-(0) | H2AlO3+H2O+3e=Al+4OH | -2.33 |
P(I)-(0) | H2PO2+e=P+2OH | -1.82 |
B(III)-(0) | H2BO3+H2O+3e=B+4OH | -1.79 |
P(III)-(0) | HPO3+2H2O+3e=P+5OH | -1.71 |
Si(IV)-(0) | SiO3+3H2O+4e=Si+6OH | -1.697 |
P(III)-(I) | HPO3+2H2O+2e=H2PO2+3OH | -1.65 |
Mn(II)-(0) | Mn(OH)2+2e=Mn+2OH | -1.56 |
Cr(III)-(0) | Cr(OH)3+3e=Cr+3OH | -1.48 |
*Zn(II)-(0) | [Zn(CN)4]+2e=Zn+4CN | -1.26 |
Zn(II)-(0) | ZnO+H2O+2e=Zn+2OH | -1.249 |
Ga(III)-(0) | H2GaO3+H2O+3e=Ga+4OH | -1.219 |
Zn(II)-(0) | ZnO2+2H2O+2e=Zn+4OH | -1.215 |
Cr(III)-(0) | CrO2+2H2O+3e=Cr+4OH | -1.2 |
Te(0)-(-I) | Te+2e=Te | -1.143 |
P(V)-(III) | PO4+2H2O+2e=HPO3+3OH | -1.05 |
*Zn(II)-(0) | [Zn(NH3)4]+2e=Zn+4NH3 | -1.04 |
*W(VI)-(0) | WO4+4H2O+6e=W+8OH | -1.01 |
*Ge(IV)-(0) | HGeO3+2H2O+4e=Ge+5OH | -1.0 |
Sn(IV)-(II) | [Sn(OH)6]+2e=HSnO2+H2O+3OH | -0.93 |
S(VI)-(IV) | SO4+H2O+2e=SO3+2OH | -0.93 |
Se(0)-(-II) | Se+2e=Se | -0.924 |
Sn(II)-(0) | HSnO2+H2O+2e=Sn+3OH | -0.909 |
P(0)-(-III) | P+3H2O+3e=PH3(g)+3OH | -0.87 |
N(V)-(IV) | 2NO3+2H2O+2e=N2O4+4OH | -0.85 |
H(I)-(0) | 2H2O+2e=H2+2OH | -0.8277 |
Cd(II)-(0) | Cd(OH)2+2e=Cd(Hg)+2OH | -0.809 |
Co(II)-(0) | Co(OH)2+2e=Co+2OH | -0.73 |
Ni(II)-(0) | Ni(OH)2+2e=Ni+2OH | -0.72 |
As(V)-(III) | AsO4+2H2O+2e=AsO2+4OH | -0.71 |
Ag(I)-(0) | Ag2S+2e=2Ag+S | -0.691 |
As(III)-(0) | AsO2+2H2O+3e=As+4OH | -0.68 |
Sb(III)-(0) | SbO2+2H2O+3e=Sb+4OH | -0.66 |
*Re(VII)-(IV) | ReO4+2H2O+3e=ReO2+4OH | -0.59 |
*Sb(V)-(III) | SbO3+H2O+2e=SbO2+2OH | -0.59 |
Re(VII)-(0) | ReO4+4H2O+7e=Re+8OH | -0.584 |
*S(IV)-(II) | 2SO3+3H2O+4e=S2O3+6OH | -0.58 |
Te(IV)-(0) | TeO3+3H2O+4e=Te+6OH | -0.57 |
Fe(III)-(II) | Fe(OH)3+e=Fe(OH)2+OH | -0.56 |
S(0)-(-II) | S+2e=S | -0.47627 |
Bi(III)-(0) | Bi2O3+3H2O+6e=2Bi+6OH | -0.6 |
N(III)-(II) | NO2+H2O+e=NO+2OH | -0.46 |
*Co(II)-(0) | [Co(NH3)6]+2e=Co+6NH3 | -0.422 |
Se(IV)-(0) | SeO3+3H2O+4e=Se+6OH | -0.366 |
Cu(I)-(0) | Cu2O+H2O+2e=2Cu+2OH | -0.360 |
Tl(I)-(0) | TlOH+e=Tl+OH | -0.34 |
*Ag(I)-(0) | [Ag(CN)2]+e=Ag+2CN | -0.31 |
Cu(II)-(0) | Cu(OH)2+2e=Cu+2OH | -0.222 |
Cr(VI)-(III) | CrO4+4H2O+3e=Cr(OH)3+5OH | -0.13 |
*Cu(I)-(0) | [Cu(NH3)2]+e=Cu+2NH3 | -0.12 |
O(0)-(-I) | O2+H2O+2e=HO2+OH | -0.076 |
Ag(I)-(0) | AgCN+e=Ag+CN | -0.017 |
N(V)-(III) | NO3+H2O+2e=NO2+2OH | 0.01 |
Se(VI)-(IV) | SeO4+H2O+2e=SeO3+2OH | 0.05 |
Pd(II)-(0) | Pd(OH)2+2e=Pd+2OH | 0.07 |
S(II,V)-(II) | S4O6+2e=2S2O3 | 0.08 |
Hg(II)-(0) | HgO+H2O+2e=Hg+2OH | 0.0977 |
Co(III)-(II) | [Co(NH3)6]+e=[Co(NH3)6] | 0.108 |
Pt(II)-(0) | Pt(OH)2+2e=Pt+2OH | 0.14 |
Co(III)-(II) | Co(OH)3+e=Co(OH)2+OH | 0.17 |
Pb(IV)-(II) | PbO2+H2O+2e=PbO+2OH | 0.247 |
I(V)-(-I) | IO3+3H2O+6e=I+6OH | 0.26 |
Cl(V)-(III) | ClO3+H2O+2e=ClO2+2OH | 0.33 |
Ag(I)-(0) | Ag2O+H2O+2e=2Ag+2OH | 0.342 |
Fe(III)-(II) | [Fe(CN)6]+e=[Fe(CN)6] | 0.358 |
Cl(VII)-(V) | ClO4+H2O+2e=ClO3+2OH | 0.36 |
*Ag(I)-(0) | [Ag(NH3)2]+e=Ag+2NH3 | 0.373 |
O(0)-(-II) | O2+2H2O+4e=4OH | 0.401 |
I(I)-(-I) | IO+H2O+2e=I+2OH | 0.485 |
*Ni(IV)-(II) | NiO2+2H2O+2e=Ni(OH)2+2OH | 0.490 |
Mn(VII)-(VI) | MnO4+e=MnO4 | 0.558 |
Mn(VII)-(IV) | MnO4+2H2O+3e=MnO2+4OH | 0.595 |
Mn(VI)-(IV) | MnO4+2H2O+2e=MnO2+4OH | 0.60 |
Ag(II)-(I) | 2AgO+H2O+2e=Ag2O+2OH | 0.607 |
Br(V)-(-I) | BrO3+3H2O+6e=Br+6OH | 0.61 |
Cl(V)-(-I) | ClO3+3H2O+6e=Cl+6OH | 0.62 |
Cl(III)-(I) | ClO2+H2O+2e=ClO+2OH | 0.66 |
I(VII)-(V) | H3IO6+2e=IO3+3OH | 0.7 |
Cl(III)-(-I) | ClO2+2H2O+4e=Cl+4OH | 0.76 |
Br(I)-(-I) | BrO+H2O+2e=Br+2OH | 0.761 |
Cl(I)-(-I) | ClO+H2O+2e=Cl+2OH | 0.841 |
*Cl(IV)-(III) | ClO2(g)+e=ClO2 | 0.95 |
O(0)-(-II) | O3+H2O+2e=O2+2OH | 1.24 |
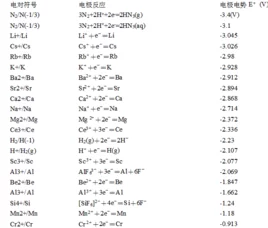
标准电极电势
半反应 | E°(V) | 来源 |
3N2(g)+2H+2e=2HN3(aq) | -3.09 | [6] |
Li+e=Li(s) | -3.0401 | [5] |
N2(g)+4H2O+2e=2NH2OH(aq)+2OH | -3.04 | [6] |
K+e=K(s) | -2.931 | [5] |
Rb+e=Rb(s) | -2.927 | [4] |
Cs+e=Cs(s) | -2.925 | [5] |
Ba+2e=Ba(s) | -2.912 | [5] |
La(OH)3(s)+3e=La(s)+3OH | -2.90 | [5] |
Sr+2e=Sr(s) | -2.899 | [5] |
Ca+2e=Ca(s) | -2.868 | [5] |
Eu+2e=Eu(s) | -2.812 | [5] |
Ra+2e=Ra(s) | -2.8 | [5] |
Na+e=Na(s) | -2.71 | [5][9] |
La+3e=La(s) | -2.379 | [5] |
Y+3e=Y(s) | -2.372 | [5] |
Mg+2e=Mg(s) | -2.372 | [5] |
ZrO(OH)2(s)+H2O+4e=Zr(s)+4OH | -2.36 | [5] |
Al(OH)4+3e=Al(s)+4OH | -2.33 | 无 |
Al(OH)3(s)+3e=Al(s)+3OH | -2.31 | 无 |
H2(g)+2e=2H | -2.25 | 无 |
Ac+3e=Ac(s) | -2.20 | 无 |
Be+2e=Be(s) | -1.85 | 无 |
U+3e=U(s) | -1.66 | [7] |
Al+3e=Al(s) | -1.66 | [9] |
Ti+2e=Ti(s) | -1.63 | [9] |
ZrO2(s)+4H+4e=Zr(s)+2H2O | -1.553 | [5] |
Zr+4e=Zr(s) | -1.45 | [5] |
TiO(s)+2H+2e=Ti(s)+H2O | -1.31 | 无 |
Ti2O3(s)+2H+2e=2TiO(s)+H2O | -1.23 | 无 |
Ti+3e=Ti(s) | -1.21 | 无 |
Te(s)+2e=Te | -1.143 | [2] |
V+2e=V(s) | -1.13 | 无 |
Nb+3e=Nb(s) | -1.099 | 无 |
Sn(s)+4H+4e=SnH4(g) | -1.07 | 无 |
Mn+2e=Mn(s) | -1.029 | [9] |
SiO2(s)+4H+4e=Si(s)+2H2O | -0.91 | 无 |
B(OH)3(aq)+3H+3e=B(s)+3H2O | -0.89 | 无 |
TiO+2H+4e=Ti(s)+H2O | -0.86 | 无 |
Bi(s)+3H+3e=BiH3 | -0.8 | 无 |
2H2O+2e=H2(g)+2OH | -0.8277 | [5] |
Zn+2e=Zn(Hg) | -0.7628 | [5] |
Zn+2e=Zn(s) | -0.7618 | [5] |
Ta2O5(s)+10H+10e=2Ta(s)+5H2O | -0.75 | 无 |
Cr+3e=Cr(s) | -0.74 | 无 |
[Au(CN)2]+e=Au(s)+2CN | -0.60 | 无 |
Ta+3e=Ta(s) | -0.6 | 无 |
PbO(s)+H2O+2e=Pb(s)+2OH | -0.58 | 无 |
2TiO2(s)+2H+2e=Ti2O3(s)+H2O | -0.56 | 无 |
Ga+3e=Ga(s) | -0.53 | 无 |
U+e=U | -0.52 | [7] |
H3PO2(aq)+H+e=P(白磷)*+2H2O | -0.508 | [5] |
H3PO3(aq)+2H+2e=H3PO2(aq)+H2O | -0.499 | [5] |
H3PO3(aq)+3H+3e=P(红磷)*+3H2O | -0.454 | [5] |
Fe+2e=Fe(s) | -0.44 | [9] |
2CO2(g)+2H+2e=HOOCCOOH(aq) | -0.43 | 无 |
Cr+e=Cr | -0.42 | 无 |
Cd+2e=Cd(s) | -0.40 | [9] |
GeO2(s)+2H+2e=GeO(s)+H2O | -0.37 | 无 |
Cu2O(s)+H2O+2e=2Cu(s)+2OH | -0.360 | [5] |
PbSO4(s)+2e=Pb(s)+SO4 | -0.3588 | [5] |
PbSO4(s)+2e=Pb(Hg)+SO4 | -0.3505 | [5] |
Eu+e=Eu | -0.35 | [7] |
In+3e=In(s) | -0.34 | [2] |
Tl+e=Tl(s) | -0.34 | [2] |
Ge(s)+4H+4e=GeH4(g) | -0.29 | 无 |
Co+2e=Co(s) | -0.28 | [5] |
H3PO4(aq)+2H+2e=H3PO3(aq)+H2O | -0.276 | [5] |
V+e=V | -0.26 | [9] |
Ni+2e=Ni(s) | -0.25 | 无 |
As(s)+3H+3e=AsH3(g) | -0.23 | [2] |
MoO2(s)+4H+4e=Mo(s)+2H2O | -0.15 | 无 |
Si(s)+4H+4e=SiH4(g) | -0.14 | 无 |
Sn+2e=Sn(s) | -0.13 | 无 |
O2(g)+H+e=HO2·(aq) | -0.13 | 无 |
Pb+2e=Pb(s) | -0.13 | [9] |
WO2(s)+4H+4e=W(s)+2H2O | -0.12 | 无 |
P(红磷)+3H+3e=PH3(g) | -0.111 | [5] |
CO2(g)+2H+2e=HCOOH(aq) | -0.11 | 无 |
Se(s)+2H+2e=H2Se(g) | -0.11 | 无 |
CO2(g)+2H+2e=CO(g)+H2O | -0.11 | 无 |
SnO(s)+2H+2e=Sn(s)+H2O | -0.10 | 无 |
SnO2(s)+2H+2e=SnO(s)+H2O | -0.09 | 无 |
WO3(aq)+6H+6e=W(s)+3H2O | -0.09 | [2] |
P(白磷)+3H+3e=PH3(g) | -0.063 | [5] |
HCOOH(aq)+2H+2e=HCHO(aq)+H2O | -0.03 | 无 |
2H+2e=H2(g) | ≡0 | 无 |
S4O6+2e=2S2O3 | +0.08 | 无 |
Fe3O4(s)+8H+8e=3Fe(s)+4H2O | +0.085 | [8] |
N2(g)+2H2O+6H+6e=2NH4OH(aq) | +0.092 | 无 |
HgO(s)+H2O+2e=Hg(l)+2OH | +0.0977 | 无 |
Cu(NH3)4+e=Cu(NH3)2+2NH3 | +0.10 | [2] |
Ru(NH3)6+e=Ru(NH3)6 | +0.10 | [7] |
N2H4(aq)+4H2O+2e=2NH4+4OH | +0.11 | 无 |
H2MoO4(aq)+6H+6e=Mo(s)+4H2O | +0.11 | 无 |
Ge+4e=Ge(s) | +0.12 | 无 |
C(s)+4H+4e=CH4(g) | +0.13 | [2] |
HCHO(aq)+2H+2e=CH3OH(aq) | +0.13 | 无 |
S(s)+2H+2e=H2S(g) | +0.14 | 无 |
Sn+2e=Sn | +0.15 | 无 |
Cu+e=Cu | +0.159 | [2] |
HSO4+3H+2e=SO2(aq)+2H2O | +0.16 | 无 |
UO2+e=UO2 | +0.163 | [7] |
SO4+4H+2e=SO2(aq)+2H2O | +0.17 | 无 |
TiO+2H+e=Ti+H2O | +0.19 | 无 |
Bi+2e=Bi | +0.2 | 无 |
SbO+2H+3e=Sb(s)+H2O | +0.20 | 无 |
H3AsO3(aq)+3H+3e=As(s)+3H2O | +0.24 | 无 |
GeO(s)+2H+2e=Ge(s)+H2O | +0.26 | 无 |
UO2+4H+e=U+2H2O | +0.273 | [7] |
Re+3e=Re(s) | +0.300 | 无 |
Bi+3e=Bi(s) | +0.32 | 无 |
VO+2H+e=V+H2O | +0.34 | 无 |
Cu+2e=Cu(s) | +0.340 | [2] |
[Fe(CN)6]+e=[Fe(CN)6] | +0.36 | 无 |
O2(g)+2H2O+4e=4OH(aq) | +0.40 | [9] |
H2MoO4+6H+3e=Mo+4H2O | +0.43 | 无 |
Bi+e=Bi(s) | +0.50 | 无 |
CH3OH(aq)+2H+2e=CH4(g)+H2O | +0.50 | 无 |
SO2(aq)+4H+4e=S(s)+2H2O | +0.50 | 无 |
Cu+e=Cu(s) | +0.520 | [2] |
CO(g)+2H+2e=C(s)+H2O | +0.52 | 无 |
I2(s)+2e=2I | +0.54 | [9] |
I3+2e=3I | +0.53 | [9] |
[AuI4]+3e=Au(s)+4I | +0.56 | 无 |
H3AsO4(aq)+2H+2e=H3AsO3(aq)+H2O | +0.56 | 无 |
[AuI2]+e=Au(s)+2I | +0.58 | 无 |
MnO4+2H2O+3e=MnO2(s)+4OH | +0.59 | 无 |
S2O3+6H+4e=2S(s)+3H2O | +0.60 | 无 |
H2MoO4(aq)+2H+2e=MoO2(s)+2H2O | +0.65 | 无 |
O2(g)+2H+2e=H2O2(aq) | +0.70 | 无 |
Tl+3e=Tl(s) | +0.72 | 无 |
PtCl6+2e=PtCl4+2Cl | +0.726 | [7] |
H2SeO3(aq)+4H+4e=Se(s)+3H2O | +0.74 | 无 |
PtCl4+2e=Pt(s)+4Cl | +0.758 | [7] |
Fe+e=Fe | +0.77 | 无 |
Ag+e=Ag(s) | +0.7996 | [5] |
Hg2+2e=2Hg(l) | +0.80 | 无 |
NO3(aq)+2H+e=NO2(g)+H2O | +0.80 | 无 |
[AuBr4]+3e=Au(s)+4Br | +0.85 | 无 |
Hg+2e=Hg(l) | +0.85 | 无 |
MnO4+H+e=HMnO4 | +0.90 | 无 |
2Hg+2e=Hg2 | +0.91 | [2] |
Pd+2e=Pd(s) | +0.915 | [7] |
[AuCl4]+3e=Au(s)+4Cl | +0.93 | 无 |
MnO2(s)+4H+e=Mn+2H2O | +0.95 | 无 |
[AuBr2]+e=Au(s)+2Br | +0.96 | 无 |
Br2(l)+2e=2Br | +1.07 | 无 |
Br2(aq)+2e=2Br | +1.09 | [9] |
IO3+5H+4e=HIO(aq)+2H2O | +1.13 | 无 |
[AuCl2]+e=Au(s)+2Cl | +1.15 | 无 |
HSeO4+3H+2e=H2SeO3(aq)+H2O | +1.15 | 无 |
Ag2O(s)+2H+2e=2Ag(s)+H2O | +1.17 | 无 |
ClO3+2H+e=ClO2(g)+H2O | +1.18 | 无 |
Pt+2e=Pt(s) | +1.188 | [7] |
ClO2(g)+H+e=HClO2(aq) | +1.19 | 无 |
2IO3+12H+10e=I2(s)+6H2O | +1.20 | 无 |
ClO4+2H+2e=ClO3+H2O | +1.20 | 无 |
O2(g)+4H+4e=2H2O | +1.23 | [9] |
MnO2(s)+4H+2e=Mn+2H2O | +1.23 | 无 |
Tl+2e=Tl | +1.25 | 无 |
Cl2(g)+2e=2Cl | +1.36 | [9] |
Cr2O7+14H+6e=2Cr+7H2O | +1.33 | 无 |
CoO2(s)+4H+e=Co+2H2O | +1.42 | 无 |
2NH3OH+H+2e=N2H5+2H2O | +1.42 | [6] |
2HIO(aq)+2H+2e=I2(s)+2H2O | +1.44 | 无 |
Ce+e=Ce | +1.44 | 无 |
BrO3+5H+4e=HBrO(aq)+2H2O | +1.45 | 无 |
β-PbO2(s)+4H+2e=Pb+2H2O | +1.460 | [2] |
α-PbO2(s)+4H+2e=Pb+2H2O | +1.468 | [2] |
2BrO3+12H+10e=Br2(l)+6H2O | +1.48 | 无 |
2ClO3+12H+10e=Cl2(g)+6H2O | +1.49 | 无 |
MnO4+8H+5e=Mn+4H2O | +1.51 | 无 |
HO2·+H+e=H2O2(aq) | +1.51 | 无 |
Au+3e=Au(s) | +1.52 | 无 |
NiO2(s)+2H+2e=Ni+2OH | +1.59 | 无 |
2HClO(aq)+2H+2e=Cl2(g)+2H2O | +1.63 | 无 |
Ag2O3(s)+6H+4e=2Ag+3H2O | +1.67 | 无 |
HClO2(aq)+2H+2e=HClO(aq)+H2O | +1.67 | 无 |
Pb+2e=Pb | +1.69 | [2] |
MnO4+4H+3e=MnO2(s)+2H2O | +1.70 | 无 |
H2O2(aq)+2H+2e=2H2O | +1.78 | 无 |
AgO(s)+2H+e=Ag+H2O | +1.77 | 无 |
Co+e=Co | +1.82 | 无 |
Au+e=Au(s) | +1.83 | [2] |
BrO4+2H+2e=BrO3+H2O | +1.85 | 无 |
Ag+e=Ag | +1.98 | [2] |
S2O8+2e=2SO4 | +2.07 | 无 |
O3(g)+2H+2e=O2(g)+H2O | +2.075 | [7] |
HMnO4+3H+2e=MnO2(s)+2H2O | +2.09 | 无 |
F2(g)+2e=2F | +2.87 | [2][9] |
F2(g)+2H+2e=2HF(aq) | +3.05 | [2] |
备注:*由-0.454和(2×(-0.499)+(-0.508))÷3=-0.502推算出。
来源
【1】Milazzo, G., Caroli, S., and Sharma, V. K. (1978). Tables of Standard Electrode Potentials (Wiley, Chichester).
【2】Bard, A. J., Parsons, R., and Jordan, J. (1985). Standard Potentials in Aqueous Solutions (Marcel Dekker, New York).
【3】Bratsch, S. G. (1989). Journal of Physical Chemistry Reference Data Vol. 18, pp. 1-21.
【4】Vanýsek, Petr (2006). "Electrochemical Series," in Handbook of Chemistry and Physics: 87th Edition (Chemical Rubber Company).
【5】Vanýsek, Petr (2007). "Electrochemical Series", in Handbook of Chemistry and Physics: 88th Edition (Chemical Rubber Company).
【6】Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements, 2nd Edition, Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
【7】Bard, A.J., Faulkner, L.R.(2001). Electrochemical Methods. Fundamentals and Applications, 2nd edition (John Wiley and Sons Inc).
【8】Marcel Pourbaix (1966). Atlas of Electrochemical Equilibria in Aqueous Solutions (NACE International, Houston, Texas; Cebelcor, Brussels).
【9】Peter Atkins (1997). Physical Chemistry, 6th edition (W.H. Freeman and Company, New York).